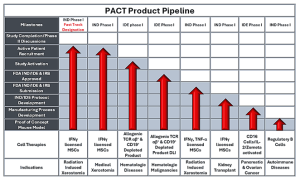
PACT specializes in conducting first-in-human clinical trials to mitigate risks associated with advanced cell therapies, utilizing UW Health’s expertise in key clinical domains. PACT empowers academic and industry partners to translate scientific discoveries to cell therapies through FDA CBER IND applications to enable Phase 1 & 2 clinical studies.
Collaborating with SMPH and UW Health, PACT has submitted for CBER approval the following applications:
- 6 Investigational New Drug (IND)
- 2 Investigational Device Exemption (IDE)
- 2 Orphan Drug Designation (ODD)
- 1 Fast Track Designation
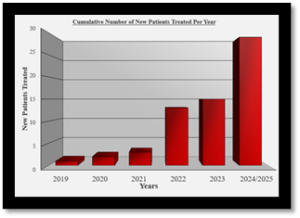
To date, more than 30 treatments have been administered, with the majority addressing conditions for which there are currently no effective therapies.
The active IND/IDE therapeutic pipe includes treatments for radiation induced xerostomia, Sjogren’s or GVHD induced xerostomia, hematologic disorders in children and young adults, and hematologic cancers. PACT’s pre-clinical work includes further studies for radiation induced xerostomia, autoimmune diseases, pancreatic and ovarian cancer treatments.