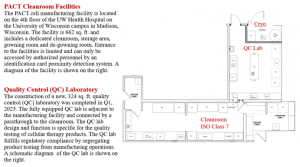
The PACT facilities consist of a Cleanroom, a Quality Control Laboratory, cryopreservation, and administrative spaces. Authorized PACT manufacturing personnel are specifically trained/qualified/certified prior to performing GMP operations.
PACT facilities, equipment and instrumentation are qualified and calibrated on a routine schedule managed by PACT personnel. Appropriate PACT facilities, storage areas, freezers, refrigerators and incubators are continuously monitored for temperature, CO2 and humidity by an automated monitoring/alarm system. Environmental monitoring, equipment, production logs, formulations, supply and cryopreservation inventory are managed by secure, GMP-compliant cellular therapy manufacturing specific software.